Chest CT superiority over COVID-19 PCR Fallacies and Weakness
Received: 28-Dec-2022, Manuscript No. 78496; Editor assigned: 31-Dec-2022, Pre QC No. 78496; Accepted Date: Dec 28, 2022 ; Reviewed: 14-Jan-2023 QC No. 78496; Revised: 18-Jan-2023, Manuscript No. 78496; Published: 25-Jan-2023, DOI: 10.24105/ejbi.2022.19.1.138-143
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact submissions@ejbi.org
Abbreviations
ARDS: Acute Respiratory Distress Syndrome COVID-19: Coronavirus Disease 2019 CT: Computed Tomography NA: Nucleic Acid NCT: Nucleic Acid Conversion Time PCR: Polymerase Chain Reaction RT-PCR: Real-time Reverse-transcriptase Polymerase Chain
Introduction
PCR in COVID-19
Identifying the priority in investigations or workups is sometimes pivotal. Coronavirus Disease 2019 (COVID-19) infection is one of the most serious worldwide infections. Discovery clarification between polymerase chain reaction (PCR) results and chest computed tomography (CT) scanning has decisive importance in diagnosis, patient isolation, treatment, and prognostication. COVID-19 clinicians had noted that some cases with positive chest CT findings might present with negative real-time reversetranscriptase polymerase chain (RT-PCR) results [1]. Evidence suggested that the pandemic of COVID‐19 may be parallel to the ability of its rapid person‐to‐person transmission [2]. However, there is specific documented treatment for COVID‐19 infection, and conventional public health measures, such as isolation, quarantine, and community containment are critical to controlling the spread [3, 4].
Study [5] for 1014 patients with suspected COVID-19 revealed that; there were 413 patients with negative RT-PCR results, of 413, 308 patients had positive chest CT findings; of 308, 48% were diagnosed as highly suspected cases, but 33% were considered probable cases [5, 6] found that five patients with typical chest CT findings suggesting COVID-19 had initial negative RTPCR results. The five patients were confirmed with COVID-19 PCR but after second or third times of swab tests for COVID-19 infection [1, 6]. Also found that the patient was confirmed with COVID-19 after the fifth time of swab test.
Significance
The initial negativity of COVID-19 PCR despite chest CT findings suggesting COVID-19 and high clinical suspicion may be a deadly established. Missed time in serial COVID-19 PCR with chest CT findings suggesting COVID-19 infection and high clinical suspicion will be lethal. This is a way to increase infection spread, patient complications, and mortality. This is due to a delay in diagnosis. Interestingly, patients with high clinical suspicion of COVID-19 but multiple negative RT-PCR results will direct the clinician that should not be taken out of isolation [1]. A constellation of the patient to COVID-19 infection exposure history, clinical presentations, laboratory workup tests, and typical chest CT findings has a crucial role in the provisional diagnosis. It is also a good directory for early isolation and treatment. This is despite repeat swab tests being considered in diagnosis for this category of patients [1].
High False Negative Rate
A false-negative case of COVID-19 infection is defined as a person with suspected infection and an initial negative result by RT-PCR test, with a positive result on a subsequent test. A high false negative rate in PCR was reported in COVID-19 infection. However, the false negative result has several adverse implications including delay in the treatment and increased risk of spread of COVID-19 infection in either hospital or community [1]. In Li Y, et al study [4], a total of 610 hospitalized patients in Wuhan city between February 2, 2020, and February 17, 2020. They reported a potentially high false negative rate of RT-PCR testing for SARS-CoV-2 in the 610 hospitalized patients. But all cases were clinically diagnosed as a COVID-19 infection during the COVID-2019 pandemic. However, recently, the higher false negative in with COVID-19 PCR result has attracted noteworthy attentiveness [7]. Certainly, patient isolation will be away out if there is a high clinical suspicion of COVID-19 with multiple negative RT-PCR results [1]. The present case reminds clinicians that a patient with high clinical suspicion of COVID-19 but multiple negative RT-PCR results should not be taken out of isolation. Repeated swab tests are helpful to make a confirmed diagnosis in this kind of patients [1].
Prolonged Nucleic Acid Conversion
This prolongation in nucleic acid conversion time (NCT) may be a risk factor in the delay of diagnosis and early isolation. NCT is defined as the period from the date of symptoms onset to the date of the first‐negative COVID‐19 RT‐PCR test result. The time for PCR positivity to negativity is defined as NCT. It is a crucial step in ceasing the isolation of patients and determining infectiousness in patients with COVID-19 [6]. The median NCT was 11 days. Patients can be divided into 2 categories according to NCT; 1. Early (if NCT <11 days) or 2. Late conversion (if NCT ≥11 days) [6]. However, in some cases, the opposite may occur. COVID-19 patients became positive RT‐PCR results after initial false‐negative results. But this is meaning these noneffective negative results in this study [8]. Recently, [6, 8] studied the characteristics of nucleic acid conversion for SARS‐CoV‐2 in 70 COVID‐19 patients. They found that 15 (21.4%) patients had got a “turn positive” of nucleic acid (NA) detection by RT‐PCR test for SARS‐CoV‐2 after two serial successive negative results. It may be interpreted as to the false negative of the RT‐PCR test and prolonged NA conversion [6, 8]. NCT was longer in patients with COVID-19 who presented with a sore throat at admission and was treated with hydroxychloroquine [6].
Interval between the Initial Negative to Positive RT-PCR
Li et al. [7] concluded that the analysis of serial RT-PCR results versus chest CT scans. And the mean interval between the initial negative to positive RT-PCR results was 5.1 days ± 1.5.
Interval between Initial Positive to Subsequent Negative RTPCR
Ai T et al. [5] that the mean interval between initial positive to succeeding negative RT-PCR results was 6.9 days ± 2.3. Of the 1014 patients, 60% (34 of 57) to 93% (14 of 15) had initial positive chest CT scans correlated to the diagnosis of COVID-19 before (or parallel to) the initial positive RT-PCR results [5].
Chest CT Scans versus RT-PCR Results Turned Negativity
Ai T et al. [5] also concluded that 24 of 57 patients (42%) showed amelioration on follow-up chest CT scans before the RT-PCR results turned negative.
False-Positivity and Misdiagnosis
A Chinese study of 610 hospitalized COVID-19 cases found that results of RT-PCR are diverse within the same patients via their diagnostic and therapeutic course. But there is hypothesized a high rate of false-positive tests7. False-positive tests were also suggested by Xiao AT et al. [8] in their study of 70 COVID-19 patients.
Long-term Positivity
Prolonged COVID-19 RT-PCR positivity for weeks or months may carry several hazards on economics, misdiagnosis, and false unneeded follow-up [9, 10]. Regrettably, many people can test positive for COVID-19 for weeks or even months. Despite that people do not have the potential to be infectious for that long, even if they test positive for COVID-19 RT-PCR, but they are improbable to transmit the COVID-19 virus to others [11].
Accuracy in the Professional Sample Selection and Low SARS-CoV-2 RT-PCR Sensitivity
Nevertheless, despite these subtle distinctions, it has been challenging for laboratory professionals to truly define the clinical sensitivity of SARS-CoV-2 RT-PCR. So, the clinician should be oriented toward the negative results in consideration of several factors. He must interpret the results in the correlation of the timing of sample collection (early postonset vs. late postonset), the type of tested samples (e.g., nasopharyngeal swab vs. throat swab), and the characteristics performance of the assay [12]. Many studies revealed that the clinical sensitivity of COVID-19 RT-PCR assays done on upper respiratory swab samples to be in the range of 60 to 70% [12].
Failure to Identify COVID-19 Infection
A false-negative RT-PCR of COVID-19 infection is a yielding factor for failure of COVID-19 diagnosis. So, RT-PCR may fail to identify infected persons [1]. This is even at this phase of the pandemic, a “test everyone” planning is improbable to be practical [12].
Time Variability
Li Y et al. [7] found that the RT-PCR results from several tests at different points were variable from the same patients during diagnosis and treatment of these patients. However, interpret the results in the correlation of the timing of sample collection as if early post-onset or late post-onset is a very important factor [12].
Strains Mutation of Coronavirus Strains
Indeed, multiple strains of the COVID-19 virus may get a very high false negative rate, fallacies, and misdiagnosis. So, the questionable debate point; is how to use a single kit for all COVID-19 virus strains?!! Otherwise, mutation of corona-virus may or may not be identified by the immunological system on successive or recurrent COVID-19 infection. Just it affects the host of the cell, promptly replication of the virus will be happening. As a result immune system will be incapable to recognize the strains of these infections [13].
Imperfect Sensitivity of RT-PCR tests
Regrettably, the sensitivity of COVID-19 RT-PCR tests is defective, with a pooled estimate of 89% (95% CI: 81%, 94%) [14]. Indeed, the clinician should know that one or more negative RT-PCR results will not rule out COVID-19 [15]. Several factors are implicated in a false RT-PCR negative result, including;
Bad quality of the sample.
Collecting the sample too early than the expected time (e.g. between exposure to SARS-CoV-2 and symptom onset, which may take up to 7 days) or late in the course of disease (eg, in the 4th week 4 post-symptom onset and beyond [16]).
Improper handling and shipping of the specimen.
And deep-seated technical factors in the test [15]. If a negative result is obtained from a patient with a high index of suspicion for COVID-19, additional specimens should be collected, and tested [15].
Late Accuracy
While the later stages of COVID-19 disease (e.g., >7 days postonset of presentations), lower respiratory tract samples (e.g. sputum, bronchoalveolar lavage fluid, or tracheal secretions) may produce higher rates of positivity and COVID-19 virus detection [17]. Sensitivity may be lower if gathered samples are technically sub-optimally or in cases with a low viral load [18].
Indirect Impacts and Adverse of False Positive RT-PCR
Indirect effects of SARS-CoV-2 RT-PCR socially may be serious. False-positive results for SARS-CoV-2 RT-PCR are not illogical. Because it may lead to loss of work, separation from family members, and unneeded psycho-social troubles.
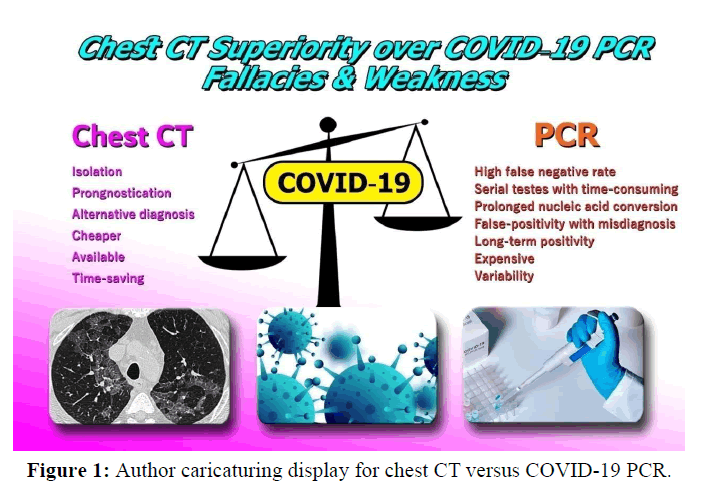
Chest CT and COVID-19
Indeed, the use of chest CT scan versus COVID-19 RT-PCR as the reference standard in the diagnosis of COVID-19 was already assessed [5] [Figure 1]. In addition, for patients with multiple RTPCR assays, the active conversion of RT-PCR results (negative to positive, positive to negative) was evaluated and compared with serial chest CT scans for those with a time interval between RT-PCR tests of 4 days or more [5].
Significance
Chest CT scanning plays an essential role in the diagnosis and follow-up of acute respiratory distress syndrome (ARDS) [19]. Chest CT scans can give advantageous information for the management of patients and detection of prognostic factors [19]. CT plays a pivotal role in the diagnosis and management of COVID-19 pneumonia [20].
In the study by [4], they found that the results indicate that in addition to the assertiveness of chest CT scan should also be used not only for diagnosis and treatment. But also for isolation, recovery/discharge, and transferring for hospitalized patients who were clinically diagnosed with COVID-19 during the current pandemic [4].
Chest CT Scanning is Valuable in Complications Detection, Prognostication, and DD
Different varieties of chest CT scanning are expected. The chest imaging findings of COVID-19 were first published in January 2020 and included bilateral lung involvement and ground-glass opacities in the majority of hospitalized patients [21]. Chest CT has a potential role in the diagnosis, detection of complications, and prognostication of coronavirus disease 2019 (COVID-19) [22] [Table 1] Chest CT is valuable to detect both alternative diagnoses and complications of COVID-19 (ARDS, pulmonary embolism, and heart failure) [22].
| S.no | Variable | Chest CT | COVID-19 PCR |
|---|---|---|---|
| 1 | Initial screening | Preferable | Unsuitable |
| 2 | Diagnosis of COVID-19 pneumonia | Very helpful | My delay the diagnosis |
| 3 | Management of COVID-19 pneumonia | Very helpful | Less helpful |
| 4 | Alternative diagnosis | Very helpful | Useless |
| 5 | Prognostication | Very good | Useless |
| 6 | Hospitalization and hospital discharge | Helpful | Less helpful |
| 7 | Follow-up | Very helpful | Useless |
| 8 | Sensitivity | High (67-100%)37 | Modest (53-88%)37 |
| 9 | Specificity | Relatively low (25-80%)37 | Higher (83-100%)37 |
| 10 | False negativity | Far | High (58%)38 |
| 11 | Strain variability and mutation | Unavailable | Available |
| 12 | Technical error | rare | More common |
| 13 | NCT | Unavailable | Prolonged |
| 14 | Cost | Cheap | Expensive (multiple assays are needed) |
| 15 | Time factor | Time-saving | Time-consuming |
Table 1: Shows comparative data between chest CT and PCR in COVID-19.
Reported COVID-19 by Chest CT in Positive and Negative PCR
Several chest CT findings have been detected in more than 70% of RT-PCR test–proven COVID-19 cases, including ground-glass opacities, vascular enlargement, bilateral chest abnormalities, lower lobe affection, and posterior tendency. Chest CT imaging is indicated in patients with moderate to severe ARDS (ie, presence of considerable pulmonary dysfunction or damage) and any pretest probability of COVID-19 infection, negative RT-PCR test results, and not readily or unavailable RT-PCR test [23].
Negative Chest CT of COVID-19 Infection and Possible other Diagnoses
A negative chest CT scan result certainly does not exclude COVID-19. The proportion of false-positive chest CT examination results is true. Several factors are implicated including overlapping imaging features with numerous other diseases, such as other viral pneumonias [23].
Importantly, the clinician should know that the chest CT is not the standard for the diagnosis of COVID-19, but its findings help suggest the diagnosis in a convenient setting. It is crucial to correlate chest CT findings with epidemiologic history, symptoms, clinical signs, and RT-PCR test results [23]. The WHO has given definitions for a suspect, probable, and confirmed cases of COVID-19 [24]. A confirmed case is defined as a patient with RT-PCR test–proven COVID-19, irrespective of clinical signs and symptoms [4].
Four Stages of Chest CT in COVID-19
Almost, there are four stages of COVID-19 pneumonia at chest CT have been reported: (a) early stage (0–5 days pos-onset of symptoms), which is characterized by either normal findings or mainly ground-glass opacities; (b) progressive stage (5–8 days pos-onset of symptoms), which is characterized by increased ground-glass opacities and crazy-paving appearance; (c) peak stage (9–13 days pos-onset of symptoms), which is characterized by progressive consolidation and (d) late stage (≥14 days posonset of symptoms), which is characterized by a gradual decrease of consolidation and ground-glass opacities, while signs of fibrosis (e.g. parenchymal bands, architectural distortion, and traction bronchiectasis) may present [25-28].
Chest CT Abnormalities that Simply Resolve After the Acute Phase
The transient turnover of lung abnormalities in COVID-19 likely parallels that of other inflammatory lung injuries19. Anyway, there are patients with chest CT abnormalities that simply resolve after the acute phase [21].
Normal chest CT Findings in Symptomatic COVID-19
However, the incidence of normal chest CT scans in symptomatic COVID-19 patients is about 10.6% (95% CI: 7.6%, 13.7%) [29]. In COVID-19 endemic regions, the chest CT findings should give a high index of the possible suspicion of COVID-19 diagnosis for the clincians [28].
Chest CT Sensitivity and Specificity
Among symptomatic adult patients, chest CT has sensitivity for the diagnosis of COVID above 90% but the specificity is lower, reportedly between 25 and 83% [18]. A meta-analysis conducted by [29], involving 16 studies and 3186 patients, confirmed the high sensitivity (92%) and low specificity (25–33%) of CT in the diagnosis of COVID-19 [29]. Interestingly, chest CT may have a key role in the diagnosis of COVID-19 in a limited number of hospitalized patients, especially [30, 31], where initial PCR testing has been indecisive, or an alternative diagnosis is being considered [18].
Added Values for Chest CT in COVID-19
In the context of the pandemic, chest CT can be used as a screening tool in symptomatic patients as it is cheaper, available, and time-saving [20]. Chest CT scanning may have a potential role as a problem-solving diagnostic tool in patients in whom RT-PCR testing remains negative, despite persistent clinical suspicion [28]. Chest CT plays a pivotal role in the diagnosis and management of COVID-19 pneumonia [32].
Preferable Initial Chest CT versus Time-Consuming and Repeated Expensive RT-PCR
Time-consuming in COVID-19 RT-PCR until results production is a decisive delayed factor in the diagnosis of the COVID-19 infection. So, COVID-19 RT-PCR usually takes several hours before the results of testing become available. Also, RT-PCR sensitivity reliably is inadequate to exclude COVID-19 due to technical errors in sampling or laboratory factors. RT-PCR testing therefore should be repeated in those individuals with a persistent clinical suspicion of COVID-19 infection [33-36]. Entirely, RT-PCR testing is rather than time-consuming, suboptimal for the rapid stratification or triage of patients is present [28].
Conclusion
The chest CT can be cheaper, rapid, time-saving, more sensitive, and advantageous in the initial screening, diagnosis, management, follow-up, and prognostication of COVID-19 pneumonia.
A COVID-19 infection PCR carries many hazards; initial negativity, delay in diagnosis, and possible complications, several tests are needed with high costs, time-consuming, and negativity due to the variability of COVID-19 strains.
Acknowledgment
I wish to thank Dr. Ameer Mekkawy, M.sc., for technical support. I want to thank my wife to save time and improving the conditions for supporting me.
References
- Chen LD, Li H, Ye YM, Wu Z, Huang YP, Zhang WL, et al. A COVID-19 patient with multiple negative results for PCR assays outside Wuhan, China: a case report. BMC Infect Dis. 2020; 20(1):1-4.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. jama. 2020; 323(13):1239-42.
- McCloskey B, Heymann DL. SARS to novel coronavirus–old lessons and new lessons. Epidemiol Infect. 2020; 148.
- Du Z, Wang L, Cauchemez S, Xu X, Wang X, Cowling BJ, et al. Risk for transportation of coronavirus disease from Wuhan to other cities in China. Emerging infectious diseases. 2020; 26(5):1049.
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020.
- Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020.
- Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability issues of RT‐PCR testing of SARS‐CoV‐2 for hospitalized patients clinically diagnosed with COVID‐19. Journal of medical virology. 2020; 92(7):903-8.
- Xiao AT, Tong YX, Zhang S. False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: rather than recurrence. Journal of medical virology. 2020.
- Canoglu K, Caliskan T, Sinmez E. Risk factors for prolonged nucleic acid conversion time in patients with COVID-19. International Journal of Health Sciences. 2022; 16(2):32.
- Joi P. How long after I get COVID-19 will I test negative?. 2021.
- Binnicker MJ. Challenges and controversies to testing for COVID-19. Journal of clinical microbiology. 2020; 58(11):e01695-20.
- Manu M. Antigen and antibody for Coronavirus RNA Vaccination Development. Op Acc J Bio Sci & Res. 1(1)-2020.
- Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020; 1.
- World Health Organization. Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance. 2020.
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. Jama. 2020; 323(22):2249-51.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020 May 12; 323(18):1843-4.
- Park JY, Freer R, Stevens R, Soneji N, Jones N. The accuracy of chest ct in the diagnosis of covid-19: An umbrella review. The Centre for Evidence-Based Medicine. 2021.
- Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Clin Respir. 2014; 23(134):519-30.
- Hefeda MM. CT chest findings in patients infected with COVID-19: review of literature. Egyptian Journal of Radiology and Nuclear Medicine. 2020; 51(1):1-5.
- Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. 2020; 27(5):609-13.
- Kwee TC, Kwee RM. Chest CT in COVID-19: what the radiologist needs to know. Radiographics. 2020; 40(7):1848.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020; 395(10223):497-506.
- World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance, 20 March 2020. World Health Organization; 2020.
- Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020.
- Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020.
- Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020; 30(6):3306-9.
- Lei P, Fan B, Mao J, Wei J, Wang P. The progression of computed tomographic (CT) images in patients with coronavirus disease (COVID-19) pneumonia: Running title: The CT progression of COVID-19 pneumonia. J Infect. 2020; 80(6):e30-1.
- Adams HJ, Kwee TC, Yakar D, Hope MD, Kwee RM. Chest CT imaging signature of coronavirus disease 2019 infection: in pursuit of the scientific evidence. Chest. 2020; 158(5):1885-95.
- Xu B, Xing Y, Peng J, Zheng Z, Tang W, Sun Y, et al. Chest CT for detecting COVID-19: a systematic review and meta-analysis of diagnostic accuracy. Eur Radiol. 2020; 30(10):5720-7.
- Hefeda MM. CT chest findings in patients infected with COVID-19: review of literature. Egyptian Journal of Radiology and Nuclear Medicine. 2020; 51(1):1-5.
- Han Y, Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID‐19): A Chinese perspective. Journal of medical virology. 2020; 92(6):639-44.
- Sharfstein JM, Becker SJ, Mello MM. Diagnostic testing for the novel coronavirus. Jama. 2020; 323(15):1437-8.
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg microbes & infect. 2020; 9(1):386-9.
- World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization; 2020.
- Kovács A, Palásti P, Veréb D, Bozsik B, Palkó A, Kincses ZT. The sensitivity and specificity of chest CT in the diagnosis of COVID-19. Eur Radiol. 2021; 31(5):2819-24.
- Pecoraro V, Negro A, Pirotti T, Trenti T. Estimate false‐negative RT‐PCR rates for SARS‐CoV‐2. A systematic review and meta‐analysis. European journal of clinical investigation. 2022; 52(2):e13706.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref